Release date:2019-03-15
JACI Volume 143, Issue 1, January 2019, Pages 78-81
[IF:13.1]
Next-generation anti–Staphylococcus aureus vaccines: A potential new therapeutic option for atopic dermatitis?https://doi.org/10.1016/j.jaci.2018.08.038
Disease severity in patients with atopic dermatitis (AD) is directly correlated with colonization by Staphylococcus aureus.1 An increasing body of evidence now also supports a role for S aureus in the pathogenesis of AD in genetically susceptible subjects.2 Increased prevalence of S aureus preceding and coinciding with AD onset in an infant cohort suggests that early skin colonization can contribute to the development of clinical AD. However, these findings only partially explain the complex role of this organism given that another birth cohort4 did not demonstrate S aureus colonization before development of infantile AD but did show a protective effect of commensal staphylococci against later development of AD.
A vaccine against S aureus in patients with AD could potentially reduce the incidence of and prevent or attenuate symptoms in a subpopulation of high-risk atopic subjects. Such a targeted approach could reduce or eliminate the role of broad-spectrum antibiotics to treat S aureus–mediated flares of AD in an era of increasing antimicrobial resistance.5 This would also avoid the suppression of potentially beneficial commensal strains of coagulase-negative staphylococci, such as Staphylococcus epidermidis and Staphylococcus hominis, which exert anti-inflammatory and selective antimicrobial activity against S aureus and are also under investigation as potential therapeutic agents in patients with AD.
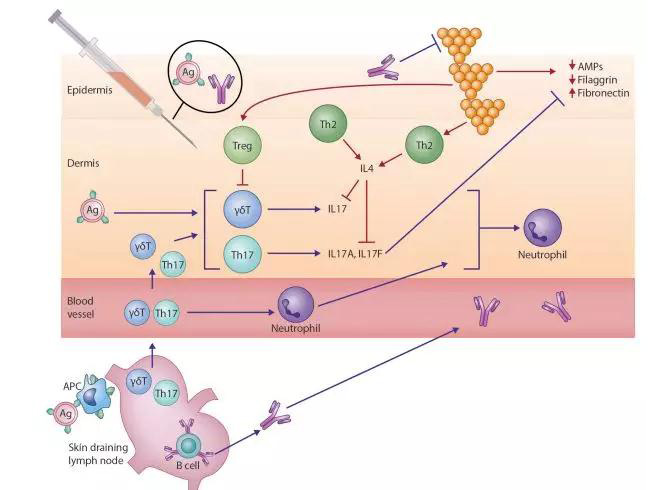
Our understanding of the cause of AD, a complex heterogenous condition, has evolved considerably in the past decade. Dichotomous views of an “outside-in” versus an “inside-out” disease process have been superseded by the recognition that AD is characterized by the interplay of both a compromised skin barrier and aberrant local and systemic immune responses. Although TH2-deviated inflammation has been long recognized as central to disease expression, TH22- and TH17-mediated inflammation are now also implicated to varying degrees across specific patient age profiles and ethnicities.7
S aureus is also now recognized as an additional key pathogenic factor in patients with AD, including its critical role in amplifying TH2-mediated responses. S aureus exploits decreased filaggrin expression resulting from inherited loss-of-function mutations and acquired through TH2 polarization and reduced antimicrobial peptide levels secondary to cutaneous dysbiosis (Fig 1).2 These factors contribute to high colonization rates in atopic skin. Once established, S aureus releases multiple virulence factors, including serine proteases, exotoxins, and lysins, such as phenol-soluble modulins. These exacerbate underlying barrier dysfunction and perpetuate endogenous dysregulated proinflammatory pathways.8S aureus cell-wall components, including peptidoglycan, amplify the TH2-driven response,8 leading to increased expression of adhesion molecules (fibronectin and fibrinogen) and reduced expression of antimicrobial peptides (human β-defensin 2 and cathelicidin antimicrobial peptide) and barrier proteins (filaggrin and loricrin).
All Author:
JulianneClowryMBabcAlan D.IrvineMD, DScabcRachel M.McLoughlinPhDd
2019-1-17 review
 杭州浙大迪迅生物基因工程有限公司
杭州浙大迪迅生物基因工程有限公司
